
BorisRabtsevich/iStock via Getty Images
Tenaya Therapeutics (NASDAQ:TNYA) is an early stage developer of heart disease therapies and a relatively new IPO that I covered briefly 2 years ago. They have a set of interesting platforms - cellular regeneration, gene therapy and precision medicine - that they are using to develop a number of molecules targeting various heart diseases. The pipeline is at an early stage, with just one molecule in the clinic, but heart disease companies are rare, and the company looks like it is doing interesting science. So we will take a look.
Tenaya was established in 2016 with IP from Gladstone Institute and UT Southwestern. The company was able to raise $50mn in a Series A financing that year. In 2019, they raised another $90mn in a series B. 2 years later, they were able to raise another $106mn in a series C financing after they published preclinical data from two programs. That same year, they launched their IPO.
The company, like I mentioned, has three platforms. The Cellular Regeneration platform delivers genes to cardiac cells using viral vectors to regenerate them. Diseases like myocardial infarction, chemotherapy-related toxicity, and viral infection which result in loss of cardiomyocytes can be targeted through this platform. The Gene Therapy platform uses AAV vectors to deliver genes to correct functional defects in heart cells. These defects could be congenital or non-genetic forms. The precision medicine platform uses "human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) as proprietary disease models and analysis of human genetics for the identification of new targets, validation of known targets, and high-throughput screening for drug discovery."
The company is very early stage and their pipeline looks thus:
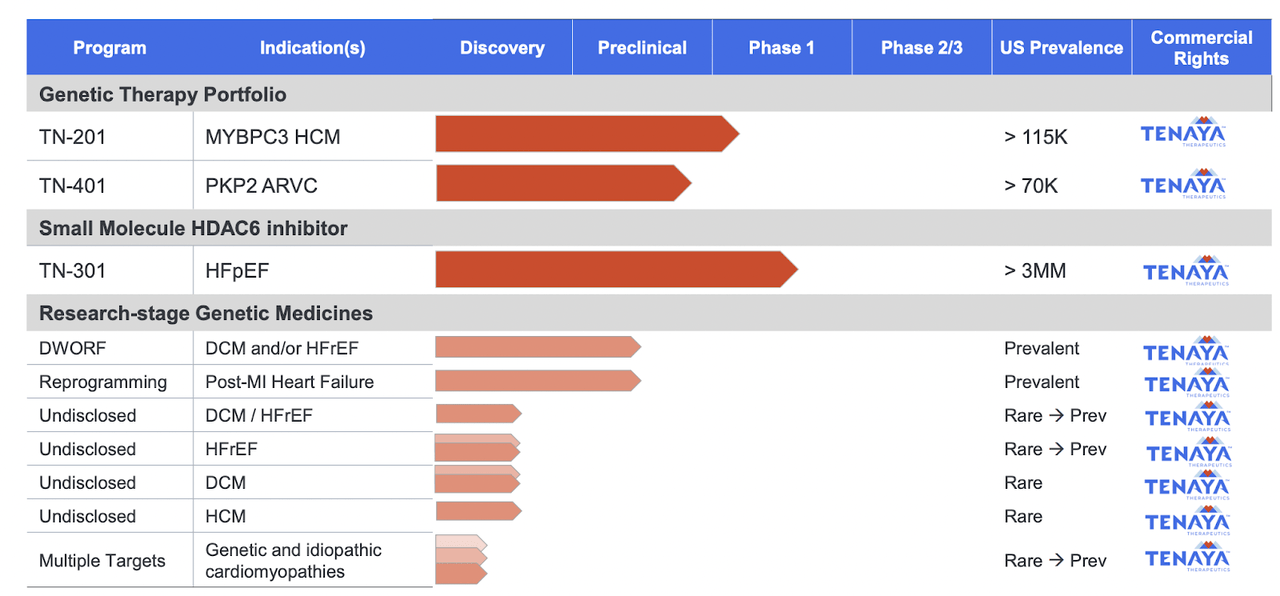
TNYA Pipeline (TNYA website)
At the time of this Corporate Presentation, they had 2 INDs approved and a third in the works. The latest stage product candidate seems to be small molecule HDAC6 inhibitor TN-301 for HFpEF or Heart Failure with preserved Ejection Fraction. This is in a phase 1 trial, however there is no listing in the registry. TN-201 also has a phase 1 trial - ongoing? - however, again there's no listing yet. TN-201 is a gene therapy targeting mutations of the MYBPC3 gene in hypertrophic cardiomyopathy (HCM). The third IND-enabled asset is TN-401, another gene therapy targeting PKP2 gene in Arrhythmogenic right ventricular cardiomyopathy.
These gene therapy assets use the AAV9 vector. AAVs have been in use for over 2 decades, and 6 gene therapies using AAVs have been FDA-approved. More than 5500 patients have been treated across 40 countries. In hundreds of trials, they have demonstrated their safety, and their long lasting transgene expression. Other important positives for AAV vectors are their low immunogenicity and ability to penetrate both dividing and nondividing cells, and so on. Some disadvantages include inability to deliver larger molecules, expensive manufacturing and so on.
As to the various diseases, MYBPC3 HCM has some 115k US patients. This genetic mutation is the most common form of inherited cardiomyopathy. There are no treatments for the underlying genetic mutation although the disease can lead to higher risks of sudden heart failures. Tenaya's treatment produces a functional copy of the MYBPC3 gene to the cardiomyocytes. These transgenes produce the MyBP-C protein which carries out normal heart function.
In preclinical trials, TN-201 demonstrated that despite a 5x increase in RNA versus wild type genes, there was no protein overexpression:
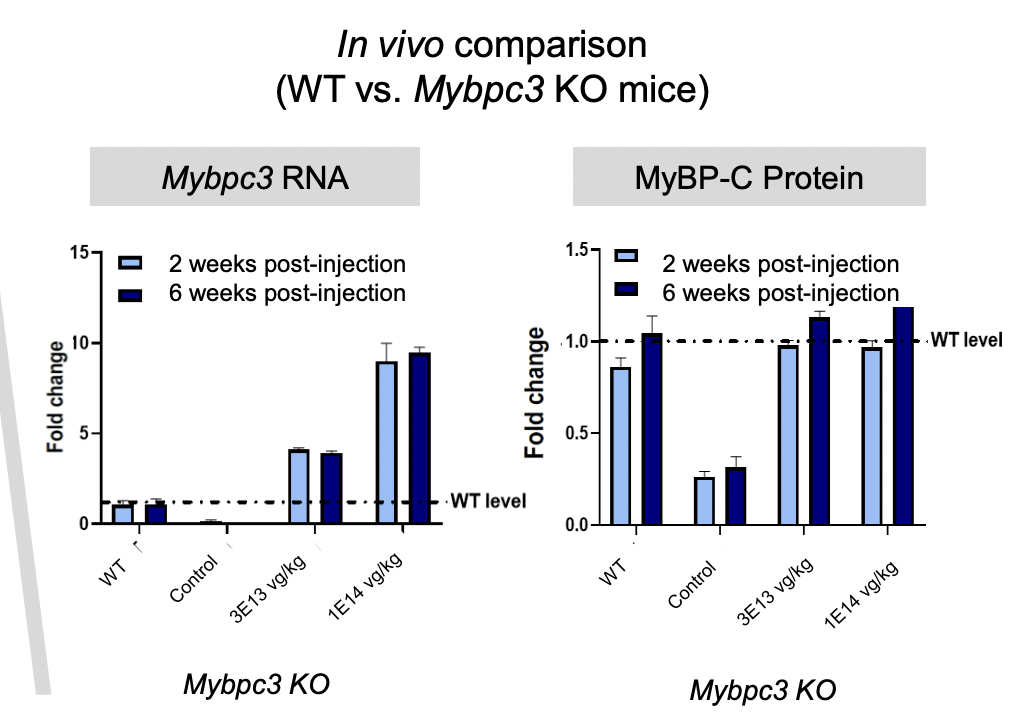
In vivo comparison (Company website)
There was also higher selectivity for the heart than other cells elsewhere in the body. Preclinical data also showed signs of efficacy in mice models. A single dose in mice demonstrated reduced hypertrophy, durable improvement in cardiac function and extended survival. The phase 1b study, informed by this preclinical data, will begin dosing in Q3. It is an open label dose escalation and dose expansion study. Initial data is expected in 2024.
The small molecule HDAC6 inhibitor TN-301 targets HFpEF. HDAC6 inhibition is an area of recent research interest in stopping the progression of this disease. In 2021, data published in Nature from a study of CKD-506 showed improvements in exercise capacity, heart function, and quality of life. Standard treatments for HFpEF have not included this modality previously. Tenaya says that in preclinical studies, TN-301 has shown a differentiated mechanism versus SGLT2 inhibitors, which are part of the arsenal against HFpEF. The company will start a randomized, placebo-controlled phase 1 SAD/MAD study with safety and tolerability as primary endpoints and PK/PD as secondary endpoints. The company says that "Dosing Commenced in Multiple-Ascending Dose Stage of First-In-Human Clinical Trial of TN-301; Data Anticipated in Second Half 2023." I still do not see anything on the registry.
Financials
TNYA has a market cap of $180mn and a cash balance of $204mn. In November, the company raised $77mn through a secondary offering. R&D expenses were $25.7 million for the fourth quarter and G&A expenses were $8.8 million. At that rate, they have a cash runway extending into 2025.
According to insider data, the company saw heavy insider buying in recent months. I was especially glad to see insiders buying the secondary in the open market.
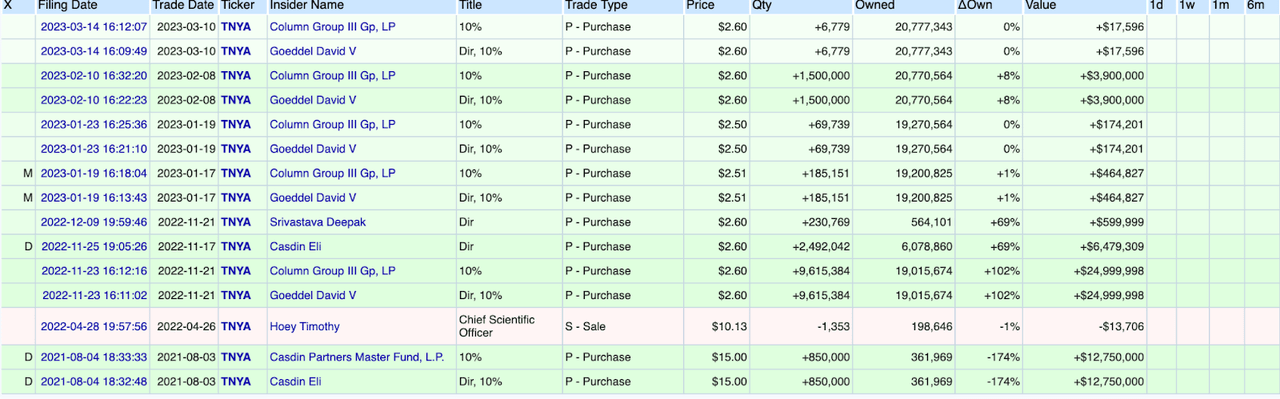
Insider transactions (openinsider.com)
The company has heavy institutional and PE/VC presence, with over 90% of the shares.
Bottomline
In the two years since I covered it last, TNYA has put together preclinical data for its assets. However, nothing has gone into the clinic, although the company has been in existence for nearly a decade, with hundreds of millions of dollars in funding available. I am afraid there's nothing to see here until we have the first proof of viability from the company. That should happen in 2024. We will take another look at that time.
About the TPT service
Thanks for reading. At the Total Pharma Tracker, we offer the following:-

Our Android app and website feature a set of tools for DIY investors, including a work-in-progress software where you can enter any ticker and get extensive curated research material.
For investors requiring hands-on support, our in-house experts go through our tools and find the best investible stocks, complete with buy/sell strategies and alerts.
Sign up now for our free trial, request access to our tools, and find out, at no cost to you, what we can do for you.
"stage" - Google News
April 10, 2023 at 01:43PM
https://ift.tt/JpdY7Hw
Tenaya Therapeutics Has Been An Early Stage Company For Too Long (NASDAQ:TNYA) - Seeking Alpha
"stage" - Google News
https://ift.tt/8xbwTPQ
https://ift.tt/gSOb89v
Bagikan Berita Ini
















0 Response to "Tenaya Therapeutics Has Been An Early Stage Company For Too Long (NASDAQ:TNYA) - Seeking Alpha"
Post a Comment